問題
| CuSO4の溶解度: |
20℃ |
|
g |
|
|
|
|
|
|
|
|
|
|
|
|
|
60℃ |
|
g |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CuSO4・5H2O=250(g/mol)
CuSO4=160(g/mol)
水200gに硫酸銅の結晶を入れて60℃の飽和溶液をつくる.
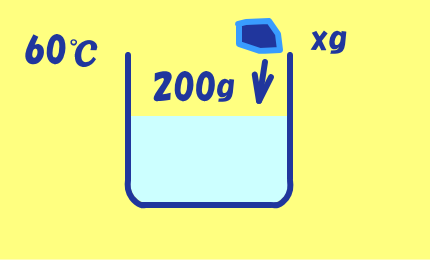
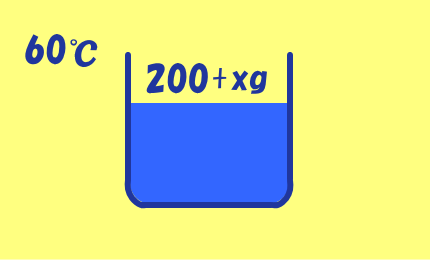
60℃の水200gにCuSO4・5H2Oの結晶がxg溶けるとすると,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x× |
—————— |
|
|
| 溶質 |
|
|
|
|
|
|
| ——— |
= |
—————————————— |
| 溶液 |
|
|
200+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
| = |
———————————— |
= |
—— |
|
|
|
|
100+ |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16 |
|
|
|
|
|
| × |
——— |
×x=(x+200)=2x+400 |
|
|
|
|
|
25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 7×16- |
|
62 |
|
|
|
|
| ——————————————— |
x= |
——— |
x= |
|
|
|
| 25 |
|
25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
×4×100 |
|
10000 |
|
|
|
| x= |
———————————————— |
= |
——————— |
|
|
|
|
62 |
|
62 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
=161.・・・≒×102g溶ける.
お疲れ様でした。「採点」ボタンを押して採点してください。
