問題
| CuSO4の溶解度: |
20℃ |
|
|
g |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60℃ |
|
|
g |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CuSO4・5H2O=250(g/mol)
CuSO4=160(g/mol)
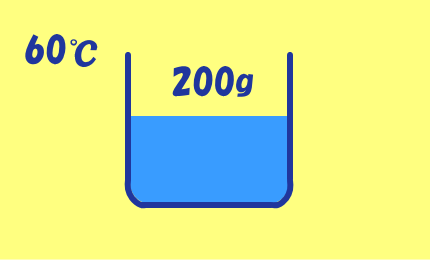
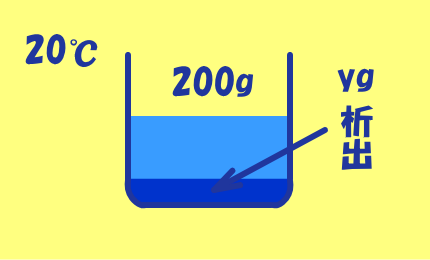
60℃の飽和溶液200gを
20℃まで冷却するとき,
CuSO4・5H2Oがy(g)析出するとすると,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
200× |
——————— |
-y× |
—————— |
|
|
|
| 溶質 |
|
|
100+40 |
|
250 |
|
|
|
| ——— |
= |
———————————————————————— |
| 溶液 |
|
200- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| = |
——————————— |
= |
—————— |
|
|
|
|
|
|
100+20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16 |
|
|
|
|
|
| (200× |
—————— |
- |
———— |
y |
)=200- |
|
|
|
|
|
|
25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2400- |
|
|
|
|
|
|
| —————— |
= |
————————————— |
|
|
|
|
|
|
| 7 |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
96- |
|
|
|
|
|
|
|
| = |
—————————— |
y= |
————— |
y |
|
|
|
|
|
25 |
|
25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25000 |
|
|
|
| y= |
—————— |
× |
—————— |
= |
—————— |
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
=50.3・・・≒(g)
お疲れ様でした。「採点」ボタンを押して採点してください。
